By: Haley S. Ball, Ph.D., Gaby L. Longsworth, Ph.D., and Michelle K. Holoubek
In the U.S., Section 101 of the Patent Act, 35 U.S.C.A. § 101, governs subject matter eligibility, and states that patents may be granted to “whoever invents or discovers any new and useful process, machine, manufacture, or composition of matter, or any new and useful improvement thereof …”
Patentable subject matter is further limited by U.S. Supreme Court precedent to exclude three categories, known as the “judicial exceptions,” from eligibility:
(1) laws of nature,
(2) natural phenomena, and
(3) abstract ideas.
In other words, inventors who make important diagnostic discoveries by, e.g., realizing key correlations between certain naturally occurring metabolites and disease states, are limited in what they can patent. The Federal Circuit’s recent decisions related to natural phenomena and abstract ideas are both challenging and frustrating for innovators in diagnostics and drug discovery.
In this article, we offer an overview of decisions since Vanda Pharma v. West-Ward Pharma (2018), key takeaways and practical tips for navigating the eligibility landmines.
Under Mayo/Alice,1 the U.S. Supreme Court provided a two-step test for determining whether subject matter is patent eligible. Step one asks whether the claimed invention is directed to one of the three judicial exceptions. If yes, step two asks whether the elements of the claimed invention, considered separately or in combination, contain an “inventive concept” that is “sufficient to ensure that the patent … amounts to significantly more than a patent upon the [judicial exception] itself.”
Previously, we discussed the evolution of diagnostic method § 101 patent eligibility in light of Vanda and Rapid Litigation Management Ltd. v. CellzDirect, Inc. (2016).2 Here, we explore how the U.S. Court of Appeals for the Federal Circuit has interpreted U.S. Supreme Court § 101 patent eligibility precedent with respect to biotechnology patents since Vanda.
Regarding method of treatment and method of preparation claims, the Federal Circuit has reaffirmed that both are patent eligible. Furthermore, the Federal Circuit has suggested that conventional methods may be used in unconventional ways to overcome patent ineligibility.
U.S. Supreme Court precedent
In 2012, in Mayo Collaborative Services v. Prometheus Laboratories, Inc., the U.S. Supreme Court held that diagnostic claims are unpatentable if they “effectively claim the underlying [diagnostic] laws of nature themselves.”
The Prometheus inventors had discovered a correlation between thiopurine metabolite levels and the toxicity/efficacy of thiopurine drugs. The claims at issue recited a three-step method of optimizing the therapeutic efficacy of a thiopurine drug treatment by:
(1) administering a thiopurine drug to a patient,
(2) measuring the patient’s resultant thiopurine metabolite levels, and
(3) adjusting the patient’s drug dose according to the patient’s resultant metabolite levels.
The Court found that the claims were merely directed to the underlying natural relationship between thiopurine drug metabolism and thiopurine metabolite levels and that, “apart from the natural laws themselves,” the other claimed elements “involve[d] well-understood, routine, conventional activity previously engaged in by researchers in the field.”
In the U.S. Supreme Court’s § 101 eligibility case, Alice Corp. v. CLS Bank International (2014), the Court reaffirmed its two-step test for determining subject matter patent eligibility when it held that conventional computer implementation does not transform an abstract idea into patent eligible subject matter.
Step lightly: Avoid step 2 of the Alice/Mayo test
In Rapid Litigation Management Ltd. v. CellzDirect, Inc. (2016), the Federal Circuit recognized that claims directed to a method of preparation are patent eligible. Recently, the Federal Circuit reaffirmed that “human-engineered” method of preparation claims are patent eligible in Illumina, Inc. v. Ariosa Diagnostics, Inc. (2020).
In addition to such claims, the Federal Circuit recognized that diagnostic claims directed to a method of treatment, rather than a diagnostic discovery itself, are patent eligible in Vanda Pharma v. West-Ward Pharma (2018).
The Federal Circuit has since reaffirmed the Vanda holding in three recent decisions: Boehringer Ingelheim Pharmaceuticals Inc. v. Mylan Pharmaceuticals Inc. (2020), Endo Pharmaceuticals Inc. v. Teva Pharmaceuticals USA, Inc. (2019), and Natural Alternatives International, Inc. v. Creative Compounds, LLC (2019).
In each of these recent decisions, the court concluded patent eligibility at step one of the Mayo/Alice test and noted that further analysis under step two was not required.
‘Human engineered’ method of preparation claims are patent eligible
In 2015, the Federal Circuit famously held in Ariosa Diagnostics, Inc. v. Sequenom, Inc. (2015), that claims directed to the detection of cell-free fetal DNA (cffDNA) in maternal blood, to identify fetal genetic abnormalities, were patent ineligible because (1) they were directed to the natural phenomena that cffDNA existed in maternal blood, and (2) the elements of isolating, amplifying, and sequencing the cffDNA were conventional, not inventive.3
By contrast, the Federal Circuit recently found Ariosa’s cffDNA claims patent eligible in Illumina, Inc. v. Ariosa Diagnostics, Inc. (2020). Ariosa’s 2020 claims differed from its 2015 claims in that they were directed to a “method of preparation,” rather than a natural phenomenon. One representative claim of the 2020 decision recited:
A method, comprising: (a) extracting DNA comprising maternal and fetal DNA fragments from a substantially cell-free sample of blood plasma or blood serum of a pregnant human female; (b) producing a fraction of the DNA extracted in (a) by: (i) size discrimination of extracellular circulatory fetal and maternal DNA fragments, and (ii) selectively removing the DNA fragments greater than approximately 300 base pairs, wherein the DNA fraction after (b) comprises extracellular circulatory fetal and maternal DNA fragments of approximately 300 base pairs and less and a plurality of genetic loci of the extracellular circulatory fetal and maternal DNA fragments; and (c) analyzing DNA fragments in the fraction of DNA produced in (b).
Although Ariosa discovered the natural phenomena that cffDNA is smaller than maternal cell-free DNA, the court found that the claims were directed to a method using a “human-engineered threshold” of less than approximately 300 base pairs, “rather than the natural size distributions of cell-free DNA,” to selectively separate and concentrate the cffDNA.
Put another way, the inventors’ method anticipated a predetermined size cutoff, rather than merely separating each sample based on its natural size distribution and removing the separated cffDNA. Accordingly, the court concluded patent eligibility at step one of the Mayo/Alice test.
Vanda-like method treatment of claims are patent eligible
The Federal Circuit also concluded patent eligibility at step one of the Mayo/Alice test in Boehringer Ingelheim Pharmaceuticals Inc. v. Mylan Pharmaceuticals Inc. (2020). The patent at issue in Boehringer Ingelheim Pharmaceuticals Inc. described treating renally impaired type 2 diabetes mellitus patients with DPP-IV inhibitors, rather than metformin. Representative claim 1 provided:
A method of treating and/or preventing metabolic diseases in a patient for whom metformin therapy is inappropriate due to at least one contraindication against metformin comprising orally administering to the patient a DPP-IV inhibitor wherein the contraindication is selected from the group consisting of: renal disease, renal impairment or renal dysfunction, unstable or acute congestive heart failure, acute or chronic metabolic acidosis, and hereditary galactose intolerance.
The Federal Circuit rejected the argument that the claims at issue were “directed to the natural law that ‘certain DPP-IV inhibitors … are metabolized by the liver rather than the kidney.’” The court analogized the case to Vanda and held that the inventors’ claims are directed to a particular method of treatment, not to the natural laws of DPP-IV metabolism.
Similarly, in Endo Pharmaceuticals Inc. v. Teva Pharmaceuticals USA, Inc. (2019), the patent at issue described treating renally impaired patients with modified oxymorphone doses dependent on patients’ measured creatinine clearance rates. Endo discovered that patients with impaired renal function, as determined by creatinine clearance rates, require lower oxymorphone doses than unimpaired patients to achieve effective pain management.
Like Boehringer Ingelheim Pharmaceuticals Inc. v. Mylan Pharmaceuticals Inc., the Federal Circuit analogized the claims at issue to those in Vanda and concluded that the claims were eligible because they are “directed to a specific method of treatment for specific patients using a specific compound at specific doses to achieve a specific outcome.”
The claims at issue in Natural Alternatives International, Inc. v. Creative Compounds, LLC (2019) were also found to be patent eligible as directed to a method of treatment. The claims described using unnatural quantities of beta-alanine to increase beta-alanylhistidine dipeptide synthesis in the muscles and other tissues, thereby increasing anaerobic working capacity and enhancing athletic performance.
Although the inventors realized the relationship between beta-alanine and beta-alanylhistidine dipeptide synthesis, the court determined that the claimed dosages directed to a method of “increas[ing] athletic performance in a way that naturally occurring beta-alanine cannot.”
In other words, the court was able to analogize the claims to Vanda because the claims were directed to a method of altering a prospective consumer’s natural state to achieve a specific outcome.
‘Withholding’ method of treatment claims are not patent eligible
By contrast, in INO Therapeutics LLC v. Praxair Distribution Inc. (2019), the Federal Circuit held that the claim at issue, directed to a method of withholding treatment, was patent ineligible as directed to a natural phenomenon with no inventive concept. The inventors discovered that newborns with left ventricular dysfunction (LVD), who are administered inhaled nitric oxide (iNO) gas, are at an increased risk of a life-threatening pulmonary edema.
Accordingly, the claim at issue recited a method of withholding iNO from newborns with LVD. The court distinguished this case from Vanda, stating that the claim did not recite “any affirmative treatment for the iNO-excluded group.” Because the court found that the claim otherwise recited only “well-understood, routine, and conventional steps,” it held that the withholding of treatment claim was patent ineligible.
Consistently ineligible — diagnostic claims
Apart from “withholding” method of treatment claims, the Federal Circuit has continued to find purely diagnostic claims patent ineligible in several recent cases: In re Board of Trustees of Leland Stanford Junior University (2021), Genetic Veterinary Sciences, Inc. v. LABOKLIN GmbH & Co. KG (2019), Cleveland Clinic Foundation v. True Health Diagnostics LLC (2019), Athena Diagnostics, Inc. v. Mayo Collaborative Services, LLC (2019), and Roche Molecular Systems, Inc. v. CEPHEID (2018).
In each instance, the court found patent ineligible claims merely reciting a natural law or an abstract idea without “a sufficiently inventive concept.”
Consistently ineligible diagnostic claims: Diagnostic claims directed to natural phenomena
In Roche Molecular Systems, Inc. v. CEPHEID (2018), the Federal Circuit determined that a “method for detecting” rifampin-resistant Mycobacterium tuberculosis (MTB) via conventional polymerase chain reaction (PCR) techniques was patent ineligible. Rifampin resistance can be conferred by mutations in the rpoB gene, which is present in various bacteria.
The Roche inventors discovered that MTB’s rpoB gene contains a fingerprint of eleven nucleotides that is absent in other bacteria. Accordingly, the claims at issue recited use of primers capable of hybridizing to MTB’s rpoB gene to amplify and detect the gene. The court observed that the primers were “indistinguishable from their corresponding nucleotide sequences on the naturally occurring DNA.”
Furthermore, the court reasoned that the hybridization of the primers to MTB’s rpoB gene was an entirely natural phenomenon. Because the claimed method employed otherwise conventional PCR techniques, the Federal Circuit concluded the claims were patent ineligible.
Similarly, in Genetic Veterinary Sciences, Inc. v. LABOKLIN GmbH & Co. KG (2019), a conventional “method for genotyping” Labrador retrievers to determine if they are genetically predisposed to the canine disease Hereditary Nasal Parakeratosis (HNPK), was deemed to be patent ineligible.
HNPK is a recessive condition; therefore, this genetic diagnosis is valuable for dog breeders. Despite the important observation correlating genotype and HNPK, the Federal Circuit determined that the claims were purely diagnostic because they were not directed to a method of treatment or preparation, and only used conventional genotyping methods.
The “method of assessing” claims at issue in Cleveland Clinic Foundation v. True Health Diagnostics LLC (2019) were likewise found to be purely diagnostic because they merely evaluated individuals’ risk of developing or having atherosclerotic cardiovascular disease according to their myeloperoxidase levels. Again, because the method of assessment comprised conventional techniques, here conventional immunoassays, the method itself was not sufficiently transformative.
The Federal Circuit’s reasoning was consistent in Athena Diagnostics, Inc. v. Mayo Collaborative Services, LLC (2019), where it decided that a “method for diagnosing neurotransmission or developmental disorders” related to muscle-specific tyrosine kinase (MuSK) comprising detecting MuSK epitopes in mammalian bodily fluids via conventional immunological assay techniques was also patent ineligible.
Consistently ineligible claims: Diagnostic claims directed to abstract ideas
In addition to diagnostic claims directed to natural phenomena, diagnostic claims directed to abstract ideas, without a sufficiently inventive concept, are also consistently patent ineligible.
In a pair of related cases titled In re Board of Trustees of Leland Stanford Junior University (2021),4 a computational method of determining haplotype phase, i.e., a computational method of determining which parent passed a specific gene to its offspring, was patent ineligible as a purely diagnostic claim directed to an abstract idea. Independent claim 1 provided:
A method for resolving haplotype phase, comprising:
- receiving allele data describing allele information regarding genotypes for a family comprising at least a mother, a father, and at least two children of the mother and the father, where the genotypes for the family contain single nucleotide variants and storing the allele data on a computer system comprising a processor and a memory;
- receiving pedigree data for the family describing information regarding a pedigree for the family and storing the pedigree data on a computer system comprising a processor and a memory;
- determining an inheritance state for the allele information described in the allele data based on identity between single nucleotide variants contained in the genotypes for the family using a Hidden Markov Model having hidden states implemented on a computer system comprising a processor and a memory, wherein the hidden states comprise inheritance states, a compression fixed error state, and a [Mendelian inheritance error]-rich fixed error state, wherein the inheritance states are maternal identical, paternal identical, identical, and non-identical; receiving transition probability data describing transition probabilities for inheritance states and storing the transition probability data on a computer system comprising a processor and a memory;
- receiving population linkage disequilibrium data and storing the population disequilibrium data on a computer system comprising a processor and a memory; determining a haplotype phase for at least one member of the family based on the pedigree data for the family, the inheritance state for the information described in the allele data, the transition probability data, and the population linkage disequilibrium data using a computer system comprising a processor and a memory; storing the haplotype phase for at least one member of the family using a computer system comprising a processor and a memory; and providing the stored haplotype phase for at least one member of the family in response to a request using a computer system comprising a processor and a memory.
Following Alice, the PTAB previously found independent claim 1 directed to an abstract idea because the claim recited “steps for receiving and analyzing information, which humans could process in their minds, or by mathematical algorithms, which are mental processes within the abstract-idea category.” Because the PTAB found that the data collection, processing, storage, and output steps “did not go beyond the well-known, routine, and conventional,” claim 1 was patent ineligible.
In line with the PTAB’s interpretation of Alice and the facts of this case, the Federal Circuit agreed that independent claim 1 merely provided haplotype phase information after a series of routine mathematical steps.
However, the court did leave open the door for eligibility in other cases where the new mathematical steps do result in some improvement to the functioning of the computer itself, noting that the court’s analysis did not consider Stanford’s arguments related to computational efficiency because they were raised for the first time on appeal.
The dependent claims, i.e., the diagnostic method claims, included the additional steps of:
(1) determining whether any stored haplotypes are associated with a disease,
(2) determining a drug for treatment, and
(3) providing the determined drug.
Because these claims were overly general and did not identify a particular disease for treatment, nor a particular treatment regimen, these claims were dissimilar to those in Vanda and were not genuine method of treatment claims.
The Federal Circuit noted that the dependent claim limitations were “drawn to making non-specific determinations of a ‘diagnosis,’ ‘drug treatment,’ and ‘prognosis’ based on the haplotype phase calculation” and did nothing more than add “apply it” language “as the Supreme Court has prohibited.”5 Accordingly, the Federal Circuit concluded that the dependent claims were also ineligible as directed to an abstract idea with no inventive concept sufficient to transform the claim.
Inventive concepts: Use conventional methods unconventionally
After determining that a claim is directed to patent ineligible subject matter under step one of Mayo/Alice, courts must then determine whether the elements of the claim, considered separately or in combination, are inventive or merely conventional.
Although the court concluded its analysis at step one in Natural Alternatives International, Inc. v. Creative Compounds, LLC, the court suggested possible step two guidance in dicta. Creative Compounds argued that “placing a natural substance into a dietary supplement for administration to a human, in order to increase the function of tissues is a conventional, well-known activity.”
In response, the court noted there was no evidence that the claimed beta-alanine dose, which was “well in excess of the normal levels of beta-alanine,” was “well-understood, routine, and conventional,” and that, absent a clear statement to the contrary, the court had no basis for making such a determination.
Similarly, in Illumina, Inc. v. Ariosa Diagnostics, Inc., the court concluded patent eligibility at step one but suggested, in dicta, that the size parameters Ariosa employed for separating cffDNA from maternal cell-free DNA were unconventional. The court observed that there was no evidence demonstrating that “thresholds of … 300 base pairs were conventional for separating different types of cell-free DNA fragments” and stated that “conventional separation technologies can be used in unconventional ways.”
Although the court’s step two analysis was not determinative to the holding of either case, these cases suggest that practitioners should consider whether their inventors’ claimed dosages or method parameters may be sufficiently unconventional to overcome patent ineligibility at Mayo/Alice step one.
In other words, practitioners should consider explaining to the PTO or the courts that, although their inventors’ techniques may be conventional, the specific manner in which they are employing the conventional technique is absent in the prior art.
For example, if the claimed doses or parameters have not been disclosed in the prior art, practitioners may try to argue that there is no basis for finding that the techniques are strictly conventional.
Conclusion
Purely diagnostic claims continue to be held patent ineligible. Practitioners should avoid typical diagnostic claim language such as: “method for detecting,”6 “method for genotyping,”7 “method of assessing,”8 “method for diagnosing,”9 and “method for resolving”10 unless they can incorporate a sufficiently inventive concept into the claims.
Method of treatment claims remain patent eligible, but must include an affirmative administration step, and the claimed treatment regimen must be as specific as possible and directed to achieving a specific outcome.
Finally, the Federal Circuit has suggested, in dicta, that inventive steps, which comprise using conventional techniques in unconventional ways, may be sufficient to transform patent ineligible subject matter at step two of the Mayo/Alice test. Namely, where applicable, practitioners should consider differentiating their manner of using a conventional technique from the prior art.
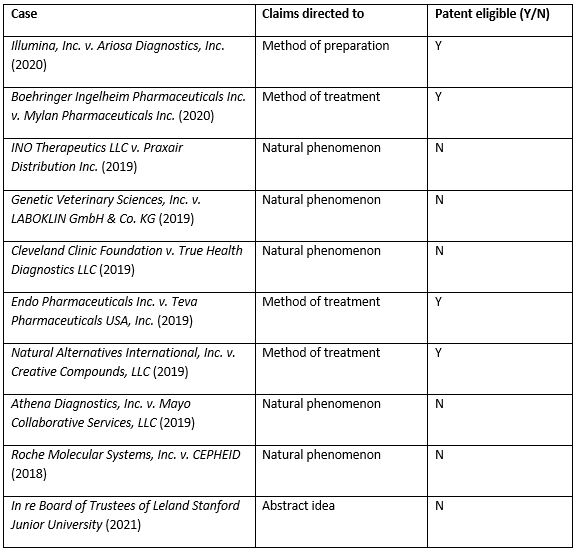
Table 1. Convenient summary table of 101 cases discussed above
Notes
1 Mayo Collaborative Servs. v. Prometheus Lab’ys, Inc., 566 U.S. 66 (2012); Alice Corp. Pty. v. CLS Bank Int’l, 573 U.S. 208 (2014).
2 Adil Moghal and Gaby L. Longsworth, “Insight: The Latest in Patenting Diagnostic Methods,” Bloomberg Law, September 24, 2018.
3 Id.
4 In re Bd. of Trustees of Leland Stanford Junior Univ., 989 F.3d 1367 (Fed. Cir. 2021).
5 Id. at 1375 (emphasis added); see Alice Corp. Pty. v. CLS Bank Int’l, 573 U.S. 208, 221 (2014).
6 Roche Molecular Sys., Inc. v. CEPHEID, 905 F.3d 1363, 1366-72 (Fed. Cir. 2018).
7 Genetic Veterinary Scis., Inc. v. LABOKLIN GmbH & Co. KG, 933 F.3d 1302, 1316 (Fed. Cir. 2019).
8 Cleveland Clinic Found. v. True Health Diagnostics LLC, 760 F. App’x 1013, 1016 (Fed. Cir. 2019).
9 Athena Diagnostics, Inc. v. Mayo Collaborative Servs. LLC, 915 F.3d 743, 747, 751-55 (Fed. Cir. 2019), cert. denied, 140 S. Ct. 855 (2020).
Related Industries
Related Services

Receive insights from the most respected practitioners of IP law, straight to your inbox.
Subscribe for Updates