35 U.S.C. § 101 precludes a patentee from obtaining more than one patent on the same invention. Courts have extended this prohibition “to preclude a second patent on an invention which ‘would have been obvious from the subject matter of the claims in the first patent, in light of the prior art.’” In re Longi, 759 F.2d 887, 893 (Fed. Cir. 1985). Thus, obviousness-type double patenting (ODP) (also known as “nonstatutory double patenting”) is a judicially created doctrine intended to prevent an improper time-wise extension of a patent right by prohibiting the issuance to a single inventor of claims in a second patent which are not “patentably distinct” from the claims of a first patent. In re Lonardo, 119 F.3d 960, 965 (Fed. Cir. 1997).
1. Why should you worry about ODP?
- Filing a terminal disclaimer (TD) to obviate an ODP rejection can reduce the patent term by limiting Patent Term Adjustment (PTA) (the term cannot extend beyond that of the earlier patent). 35 U.S.C. §§ 154(b)(2) and 253. Patents having the same earliest effective filing date may have different patent terms due to different PTA. In some instances, PTA can be more beneficial than Patent Term Extension (PTE), as it extends the term of all the claims in the patent.
- If the first (earlier) patent has expired, a TD cannot be filed and the second (later) patent will be invalid for ODP.
- Note: Filing a TD does not affect PTE obtained under 35 U.S.C. § 156. Merck & Co. v. Hi-Tech Pharmacal Co., Inc., 482 F.3d 1317 (Fed. Cir. 2007); see also Novartis AG v. Ezra Ventures LLC, 909 F.3d 1367, 1373 (Fed. Cir. 2018). However, any PTA time awarded to a patent subject to a terminal disclaimer will be limited by the expiration date of the reference patent, and patent term that is extended by PTA may affect the double patenting analysis under Gilead, infra. 35 U.S.C. § 154(b)(2)(B); see also Magna Electronics, Inc. v. TRW Automotive Holdings Corp., 2015 WL 11430786 (W.D. Mich. 2015).
2. When may ODP issues arise?
- During prosecution – The claims of an application can be rejected for ODP in view of claims of patents or applications that have at least one common inventor, that are commonly assigned/owned or non-commonly assigned/owned but subject to a joint research agreement as set forth in 35 U.S.C. § 103(c)(2)(3). See MPEP § 804 and In re Hubbell, 709 F.3d 1140 (Fed Cir. 2013).
- In litigation – ODP is an affirmative defense as it is a ground for invalidating one or more claims of a patent. See e.g., Symbol Techs., Inc. v. Opticon, Inc., 935 F.2d 1569, 1580 (Fed. Cir. 1991); Geneva Pharms. Inc. v. GlaxoSmithKline PLC, 349 F.3d 1373, 1377-78 (Fed. Cir. 2003).
3. Standard for ODP
- The claims of the second patent or application are not distinct (anticipated or obvious) in view of the claims of the first patent or application.
- The “one way” and “two way” tests:
- The one way test (default):
- Whether the claim at issue is patentably distinct over the earlier reference claim.
- The two way test (rare):
- Applies only in cases where the applicant could not have filed the claims in a single application and there is administrative (U.S. Patent and Trademark Office) delay.
- Compares the patentable distinctness of both the later claim over the earlier claim and the earlier claim over the later claim.
- The one way test (default):
- Obviousness analysis under ODP is analogous to an obviousness analysis under 35 U.S.C. § 103 except that:
- The first patent or application is not considered prior art. But reference to the specification of the first patent or application may be appropriate, e.g., for claim construction. In re Vogel, 442 F.2d 438, 441-442 (C.C.P.A. 1970).
- Lead compound analysis is not required in cases involving claimed chemical compounds. Otsuka Pharmaceuticals Co., Ltd. v. Sandoz, Inc., 678 F.3d 1280, 1298 (Fed. Cir. 2012).
- A later claim in a patent to a method of treatment using a compound is not patentably distinct from a claim to the identical compound in a first patent disclosing the identical use. See Geneva Pharmaceuticals, Inc. v. GlaxoSmithKline PLC, 349 F.3d 1373 (Fed. Cir. 2003); Pfizer, Inc. v. Teva Pharmaceuticals USA, Inc., 518 F.3d 1353, 1363 (Fed. Cir. 2008); Sun Pharmaceutical Industries, Ltd. v. Eli Lilly & Co., 611 F.3d 1381 (Fed. Cir. 2010).
- A patent that issues after but expires before another patent may qualify as a double patenting reference for that other, later expiring patent. See Gilead Sciences, Inc. v. Natco Pharma Ltd., 753 F.3d 1208 (Fed. Cir. 2014).
- The two patents can be from the same family and have a different expiration date due to PTA. Magna Elecs., Inc. v. TRW Automotive Holdings Corp. (W.D. Mich. December 10, 2015).
- However, an earlier expiring post-GATT patent cannot be used as a reference against a later expiring pre-GATT patent. Novartis Pharmaceuticals Corp. v. Breckenridge Pharmaceutical, Inc., 909 F.3d 1355 (Fed. Cir. 2018) (applying “pre-URAA obviousness-type double patenting practice” and holding that “to require patent holders to truncate any portion of the statutorily-assigned term of a pre-URAA patent that extends beyond the term of a post-URAA patent would be inconsistent with the URAA transition statute”); but see Janssen Biotech, Inc. v. Celltrion Healthcare Co. Ltd., (D. Mass. September 28, 2016), appeal dismissed, No. 17-1120, 2018 WL 2072723 (Fed. Cir. 2018) in view of In re Janssen Biotech, Inc., 880 F.3d. 1315 (Fed. Cir. 2018) (affirming the rejection of claims 1-7 of the subject patent under the doctrine of obviousness-type double patenting on other grounds).
- Case law appears to be settled regarding whether secondary indicia should be considered in an ODP analysis.
- Evidence of secondary considerations should be considered, when offered, in an ODP analysis. See Otsuka Pharmaceuticals Co., Ltd. v. Sandoz, Inc., 678 F.3d 1280, 1298 (Fed. Cir. 2012); Eli Lilly and Company v. Teva Parenteral Medicines, Inc., 689 F.3d 1368, 1378 (Fed. Cir. 2012); Abbvie Inc. v. Mathilda & Terence Kennedy Inst. of Rheumatology Trust, 764 F.3d 1366, 1372 (Fed. Cir. 2014).
4. How can you overcome ODP during prosecution?
- Argue that the claims are patentably distinct from each other.
- File a TD.
- Affirmatively disclaim any term of the second patent beyond the term of the first patent. 35 U.S.C. § 253 and 37 C.F.R. § 1.321; In re Longi, 759 F.2d at 894; Ortho Pharm. Corp. v. Smith, 959 F.2d 936, 980 (Fed. Cir. 1992).
- Patents linked by a TD will only be enforced while commonly owned.
- Signed by an owner (in part or in entirety) or an attorney or agent of record. 37 C.F.R. § 1.321.
- Filing a TD requires common ownership. In re Hubbell, 709 F.3d 1140 (Fed. Cir. 2013).
- At least one district court has held that patents assigned to two wholly owned subsidiaries are not commonly owned by the parent company for purposes of satisfying requirements of TD. See Email Link Corp. v. Treasure Island, LLC, No. 2:11-cv-01433-ECRGWF (D. Nev. Sept. 25, 2012).
- However, the U.S. Patent and Trademark Office appears to consider the above situation to be commonly owned. See MPEP §§ 1490, 706.02(I)(2).
- Need to disclaim the entire patent, not just the claims at issue. See Eli Lilly & Co. v. Barr Labs., Inc., 251 F.3d 955 (Fed. Cir. 2001).
5. How can you overcome ODP post-issuance or in litigation?
- If patents can trace their lineage back to a common parent which was subject to a restriction requirement (RRQ), then 35 U.S.C. § 121 (“safe harbor”) may prevent an ODP challenge.
- Safe harbor is only available when there was a RRQ. Bristol-Myers Squibb Co. v. Pharmachemie B.V., 361 F.3d 1343, 1347 (Fed. Cir. 2004).
- Safe harbor is only available to divisional applications, not continuations or continuations-in-part. Pfizer Inc. v. Teva Pharmaceuticals Inc., 518 F.3d 1353 (Fed. Cir. 2008); Amgen Inc. v. F. Hoffman-La Roche Ltd., 580 F.3d 1340 (Fed. Cir. 2009).
- Consonance must be maintained (the line of demarcation between the inventions identified in the RRQ). Symbol Techs., Inc. v. Opticon, Inc., 935 F.2d 1569, 1579 (Fed. Cir. 1991); Gerber Garment Tech., Inc. v. Lectra Sys., Inc., 916 F.2d 683, 688 (Fed. Cir. 1990).
- Requirement for election of species creates a restriction if no generic claim is found allowable. St. Jude Medical, Inc. v. Access Closure, Inc., 729 F.3d 1369 (Fed. Cir. 2013).
- For safe harbor to apply, the actual filing date (instead of the effective filing date) of the divisional application must be before the issuance of the patent on the application that is subjected to the restriction requirement. Ex parte Sauerberg, decision of the Patent Trial and Appeal Board, Application No. 14/016,442 (Jan. 12, 2017).
- File a TD.
- A TD can be filed at any time except after the earlier-issued patent has expired. See Boehringer Ingelheim Int’l GmbH v. Barr Labs., Inc., 592 F.3d 1340, 1347 (Fed. Cir. 2010).
- Consider filing a statutory disclaimer disclaiming only the claims challenged under ODP. 35 U.S.C. § 253(a).
- 35 U.S.C. § 253(a) authorizes disclaiming “any complete claim.”
- May not impact patent term.
- Consider filing a reissue application in the reference patent to cancel or otherwise amend the cited claims (reference patent must be eligible for reissue; see Sanofi-Aventis U.S., LLC v. Dr. Reddy’s Laboratories, Inc., Nos. 2018-1804; 1808; 1809 (Fed. Cir. 2019)).
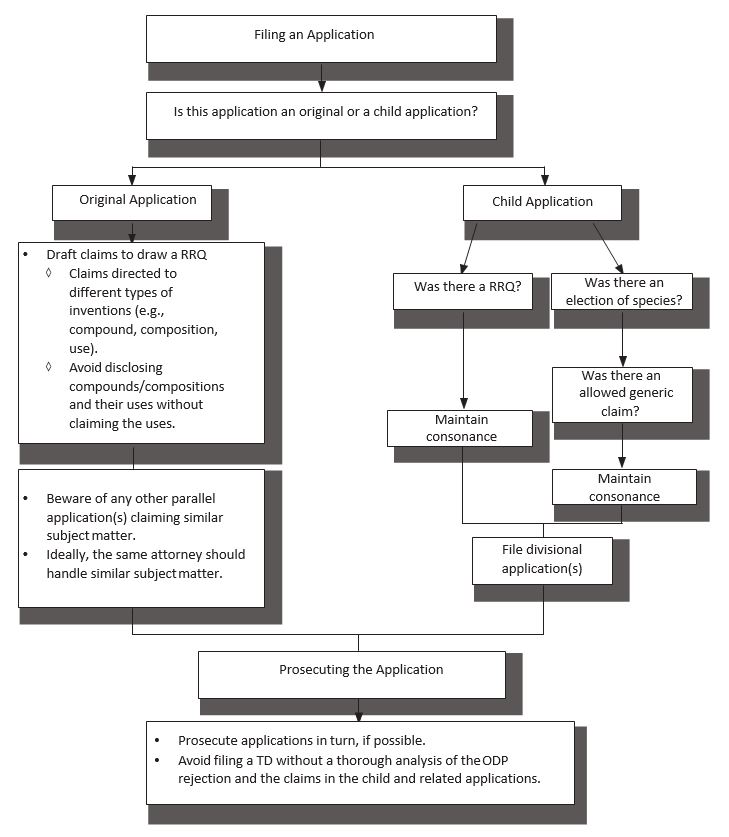
6. ODP Best Practices
- Before filing a patent application:
- Is this an original or a child application?
- Original application: draft claims to draw a RRQ.
- Child applications: file divisional applications before issuance of an original patent and maintain consonance; or, for serial continuing applications, consider filing claims identical to the full set of restricted claims in the parent application to induce an identical RRQ in each subsequent case.
- Is this an original or a child application?
- Filing the application:
- Incorrectly calling a divisional a continuation may lead to a loss of safe harbor protection.
- Prosecuting the application:
- Prosecute applications in turn, if possible.
- Beware of later filed, earlier issued patents in related families.
- E.g., if the first patent has generic claims and the second patent has species claims, the second patent may issue earlier and create ODP issues. PTA may be shortened or lost.
- Avoid filing a TD to overcome ODP without a thorough analysis of the relevant claims.
- If only some of the claims are rejected under ODP, consider splitting the claims. E.g. let the non-rejected claims issue and pursue the rejected claims in a separate application.
- A TD may not be nullified once it is filed (e.g., by arguing a TD was improper because the required fee was not paid). President and Fellows of Harvard College v. Lee, 589 Fed. Appx. 982 (Fed. Cir. 2014).
- An incorrectly filed TD is not an “error” correctable by reissue. In re Dinsmore, 757 F.3d 1343 (Fed. Cir. 2014).
This article appeared in the 2020 Patent Prosecution Tool Kit.
Related Services

Receive insights from the most respected practitioners of IP law, straight to your inbox.
Subscribe for Updates