An essential part of U.S. patent prosecution is the duty of disclosure, which requires the disclosure of all known information that is material to patentability. 37 C.F.R. § 1.56.
Who Has a Duty of Disclosure?
- Anyone substantively involved in preparation or prosecution of an application, including:
- Inventor(s)
- Attorneys, agents, and paralegals
- In-house patent counsel
Suggested Composition of an IDS Filing
- Information Disclosure Statement (IDS) pleading;
- Form PTO/SB/08a and/or PTO/SB/08b;
- Certifications (37 C.F.R. § 1.97(e)) and/or PTA safe harbor statements (37 C.F.R. § 1.704(d));
- Copies of the cited documents, if required;
- Fee payment, if required; and
- Other requests or petitions, if required (e.g., request for continued examination, petition to withdraw from issue).
Special Considerations for an IDS Pleading
- Explain:
- Materials that may be relevant to patentability are being submitted.
- The prosecution status of the application (Before examination? After first Office action? After final rejection?) and under which rule the IDS is filed.
- What copies of the cited documents are/are not being submitted.
- No need to submit:
- U.S. patents and published applications. 37 C.F.R. § 1.98(a)(2)(ii);
- Documents cited by or submitted to the U.S. Patent and Trademark Office (PTO) in an IDS filed in an earlier U.S. nonprovisional application to which the current application claims benefit; or
- Unpublished U.S. applications stored in the PTO’s Image File Wrapper system. Official Gazette, October 19, 2004.
- Any related information or matters that you desire the examiner consider:
Special Considerations for Form PTO/SB/08a
- Form PTO/SB/08a = for citing patents and published applications
- When a patent or published application is in a foreign language, it is preferable to provide an English language translation.
- Human translations are preferred if possible.
- Machine translations are often available otherwise – check website of WIPO, EPO, JPO, KIPO, SIPO, etc.
- Tip: Is the foreign language document the foreign national phase of a PCT application that was published in English?
- If so, then also submit the publication of the English language PCT publication since that is either a human translation or the original language of the application.
- Explain the relationship between the foreign language patent document and the PCT publication in the IDS pleading.
- For example, state that the PCT publication is believed to be a translation of “at least” the specification and figures of the foreign language patent document.
- Note: The claims in the foreign language patent document may be different than they are in the PCT publication.
- List the foreign language patent document and the PCT publication separately on the IDS forms.
- Tip: Is the foreign language patent document the foreign national phase of a PCT application that was not published in English, but which entered national phase in a country in which it was published in English (e.g., the U.S.)?
- If so, then also list and/or submit the English language publication.
- Explain the relationship between the foreign language patent document and the English publication in the IDS pleading.
- For example, state that the English publication is believed to be a translation of “at least” the specification and figures of the foreign language patent document.
- Note: The claims in the foreign language patent document may be different than they were in the English publication.
- Explain that the foreign language patent document and the English publication are national phase applications that arose from the same PCT application
- List the foreign language patent document and the English publication separately on the IDS forms.
- If the English publication is a U.S. application, attach a copy to the foreign language patent document as a courtesy to the examiner, even though it isn’t required.
- If no translations of the foreign document are available, a statement of relevance must be submitted.
- Alternatively, if the foreign language document was cited in a search report by a foreign patent office in a counterpart foreign application, you can submit an English language version of the search report that indicates the degree of relevance of the document.
- A copy of the English language version of the search report must be submitted. MPEP 609.04(a)(III).
Special Considerations for Form PTO/SB/08b
- Form PTO/SB/08b = for citing all other documents or other information, including unpublished U.S. patent applications.
- The citation should include author, title of the article, title of the item (book, journal, etc.), date, page(s), volume-issue number(s) and, for books, the publisher.
- Foreign language documents must be submitted with an English language translation and/or a statement of relevance.
- You can prepare your own statement of relevance, or you can submit an English language version of a search report that lists the foreign document, as explained above.
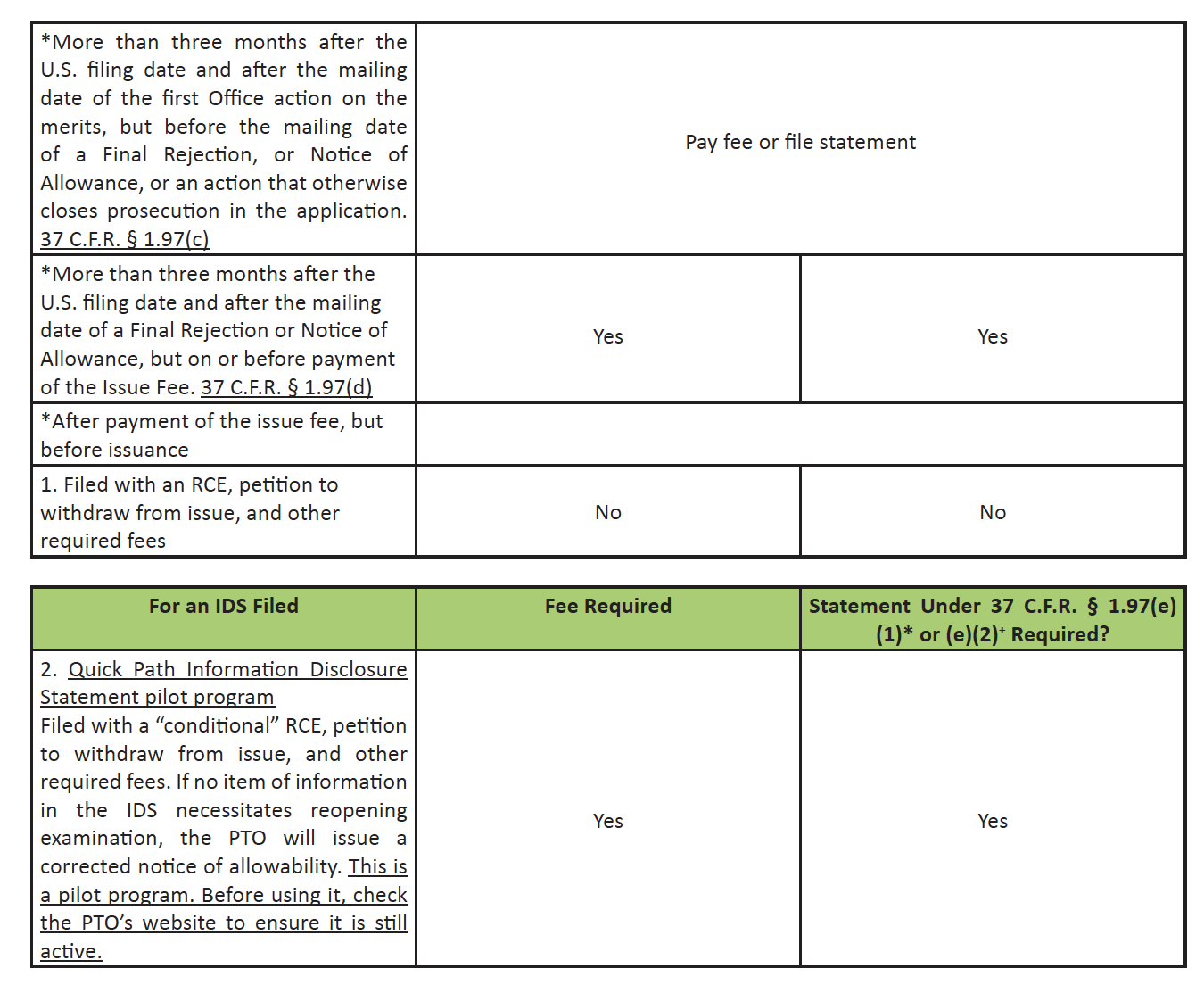
Timing of Filing an IDS
* – “I hereby state that each item of information contained in this Information Disclosure Statement was first cited in any communication from a foreign patent office in a counterpart foreign application not more than three months prior to the filing of this Information Disclosure Statement.” 37 C.F.R. § 1.97(e)(1).
# – $240 for a large entity applicant, $120 for a small entity applicant, and $60 for a micro entity applicant.
+ – “I hereby state that no item of information in this Information Disclosure Statement was cited in a communication from a foreign patent office in a counterpart foreign application and, to my knowledge after making reasonable inquiry, was known to any individual designated in 37 C.F.R. § 1.56(c) more than three months prior to the filing of this Information Disclosure Statement.” 37 C.F.R. § 1.97(e)(2).
Special Considerations for a Statement Under 37 C.F.R. § 1.97(e)
- Statement refers to a “counterpart” foreign application.Be careful when making this statement because not all “related” foreign applications are counterpart foreign applications.
- A counterpart foreign application has a specific meaning:
- “The term counterpart foreign patent application means that a claim for priority [the PTO means foreign priority] has been made in either the U.S. application or a foreign application based on the other, or that the disclosures of the U.S. and foreign patent applications are substantively identical (e.g., an application filed in the European Patent Office claiming the same U.K. priority as claimed in the U.S. application).” (MPEP 609.04(b) (V)).
- A U.S. or English language counterpart of a foreign patent or application cited by a foreign patent office can qualify the IDS for certification under 37 C.F.R. § 1.97(e)(1).
- “Some applicants submit information disclosure statements to the PTO which list and include copies of both the particular patent cited in the foreign patent office communication and the related United States or other English language patent from the family list. Since this is to be encouraged, the United States or other English language patent will be construed as being cited by the foreign patent office for purposes of a statement under 37 CFR 1.97(e)(1).” (MPEP 609.04(b)(V)).
- For example,
- You have a counterpart application in Japan.
- The Japanese examiner cites a published Japanese application, JP 2010-xxxxx, against your Japanese application.
- JP 2010-xxxxx is the publication of the Japanese national phase application that arose from PCT/US2010/yyyyyy.
- PCT/US2010/yyyyyy was published in English.
- You are after final rejection in the counterpart U.S. application.
- You can file the IDS and certify under 37 C.F.R. § 1.97(e)(1) not only JP 2010-xxxxx but also PCT/US2010/yyyyyy.
- A machine translation of an office action from the EPO, SIPO, KIPO and the JPO may be available on the application’s Global Dossier (which can be reached through PAIR). However, especially with foreign search reports, always check the original language document to ensure the machine translation correctly lists all the cited art. For example, we have noticed that with supplemental search reports from CNIPA (formerly SIPO), that are on the Global Dossier (GD), the search report often shows only the patent documents that were on the search report. The non-patent documents do not show up on the GD version of the search report. Obtain a copy of the office action/search report from your foreign associate and confirm that you have a list of all the documents cited by the foreign patent office.
- For example,
- “Some applicants submit information disclosure statements to the PTO which list and include copies of both the particular patent cited in the foreign patent office communication and the related United States or other English language patent from the family list. Since this is to be encouraged, the United States or other English language patent will be construed as being cited by the foreign patent office for purposes of a statement under 37 CFR 1.97(e)(1).” (MPEP 609.04(b)(V)).
Establish an IDS Process
- Docket reminders to consider filing an IDS:
- With or shortly after the filing of any new nonprovisional application;
- When a new search report or Office action is received in a related application; and
- Before payment of the issue fee.
- Keep a well-organized spreadsheet of cited documents in all related applications for more complex matters.
- When replying to an Office action, check that the examiner considered all previously submitted IDS’s and consider whether any new documents should be cited.
- When filing an IDS in the U.S., consider whether the documents should also be cited in any related applications in foreign countries having a duty of disclosure.
Special Considerations for Patent Term Adjustment
- Avoid filing an IDS by itself during examination.
- Considered “failure of the applicant to engage in reasonable efforts to conclude processing or examination of an application,” which is counted against a patent term adjustment (PTA) to which a case may be entitled. 37 C.F.R. § 1.704.
- An IDS filed alone after a reply to a restriction requirement and before a first Office action on the merits is considered applicant delay counted against PTA. Gilead Sciences Inc. v. Lee, 778 F.3d 1341 (Fed. Cir. 2015). But, an IDS filed alone after a restriction requirement is issued and before a reply to the restriction requirement is filed, is not considered applicant delay counted against PTA.
- An IDS filed by itself before the mailing of a first office action after the filing of an RCE did not count against PTA from the date of filing of the RCE until the filing of the IDS. Supernus Pharm., Inc. v. Iancu, 913 F.3d 1351 (Fed. Cir. 2019). The cited documents were related to an EP opposition that was not filed at the EPO until 546 days after the RCE was filed at the USPTO. Supernus filed an IDS citing the opposition documents 646 days after the RCE was filed. The USPTO subtracted 646 days of PTA. Supernus argued that it was entitled to at least 546 of the 646 days of PTA reduction because the IDS citing the opposition documents could not have been filed before that time. The Federal Circuit agreed and found that the USPTO may not count as applicant delay a period of time during which there was no action that the applicant could take to conclude prosecution of the patent.
- Request PTA Reconsideration: In view of the Supernus decision, The USPTO published a “Notice” that, effective May 9, 2019, a patentee who disagrees as to whether the USPTO’s calculation of the period of PTA reduction exceeds the period of time during which the patentee failed to engage in reasonable efforts to conclude prosecution must raise the issue and provide relevant information in a timely request for reconsideration of the PTA (84 Fed. Reg. 20343 (May 9, 2019). The problem is that the event from which the Federal Circuit measured the beginning of the PTA reduction in Supernus was a notice from a foreign authority. That type of event is not recorded in the USPTO PALM system. Therefore, the burden is on the applicant to bring such events to the USPTO’s attention and request reconsideration of the PTA calculation.
- The USPTO published a Notice of Proposed Rulemaking to align PTO rules with the decision in Supernus (84 Fed. Reg. 53090 (October 8, 2019). The comment period closed December 3, 2019. The proposed rule revises the period of reduction of PTA in 37 C.F.R. § 1.704 for the following events: deferral of issuance of a patent, abandonment of an application, submission of a preliminary amendment, submission of papers after a PTAB or Federal court decision and submission of papers after a notice of allowance. The propose rule specifies a period of reduction corresponding to “the period from the beginning to the end of the applicant’s failure to engage in reasonable efforts to conclude prosecution.”
- Safe Harbor Provisions: 37 C.F.R. § 1.704(d)(i) and (d)(ii)
- PTA is not subtracted if the information:
- Was first cited in a communication from the PTO or a patent office in a counterpart foreign or international application, and this communication was not received more than 30 days prior to the filing of the IDS (37 C.F.R. § 1.704(d)(i)); or
- Is a communication that was issued by the PTO or a patent office in a counterpart foreign or international application, and this communication was not received more than 30 days prior to the filing of the information disclosure statement. (37 C.F.R. § 1.704(d)(ii))
- To take advantage of these safe harbor provisions, be sure to clearly label the pleading as including a statement under 37 C.F.R. § 1.704(d)(i) or (d)(ii) (or use the USPTO forms below) and actually make the appropriate statement(s).
- PTA is not subtracted if the information:
- USPTO safe harbor forms
- To make an IDS “safe harbor” statement and to request PTA recalculation based on the safe harbor statement
- Use the USPTO’s “Interim Procedure” for requesting recalculation of PTA with respect to IDS’s that are accompanied by a safe harbor statement under 37 C.F.R. § 1.704(d)(i) or (d)(ii).(83 Fed. Reg. 55102 (November 2, 2018)).
- Use form PTO/SB/133 to make a safe harbor statement under 37 C.F.R. § 1.704(d). Important: this form will not satisfy the requirement of 37 CFR 1.97(e).
- Use form PTO/SB/134 to request recalculation of PTA in view of having made a safe harbor statement under 37 CFR 1.704(d). This form must be filed within the time period set forth in 37 C.F.R. § 1.705(b).
- Both forms are available at (https://www.uspto.gov/patent/patents-forms). The USPTO PALM system is being updated to automatically include these forms when calculating PTA (still in the future).
- To make an IDS “safe harbor” statement and to request PTA recalculation based on the safe harbor statement
Special Considerations for Continuing Applications
- The examiner will consider information considered by the PTO in a parent application when examining a continuation, divisional or continuation-in-part application. MPEP 609.02.
- This information need not be resubmitted, unless the applicant desires the information to be printed on the patent.
- However, when filing a continuing application that claims benefit to an international application, documents cited in the international search report (ISR) and/or international preliminary report on patentability (IPRP) should be submitted.
Special Considerations for National Phase Applications
- Documents cited in an ISR should be considered when Form PCT/DO/EO/903 indicates that the ISR and copies of the cited documents are present in the national stage file. MPEP 609.03.
- ISR, IPRP and their translations
- English language translations of the ISR and IPRP/Written Opinion are available for download directly from WIPO’s website.
- If the English language translation of the IPRP/Written Opinion is not available when the IDS is filed, docket to obtain it and submit it in a supplemental IDS as a courtesy to the examiner if WIPO has not automatically sent it to the PTO.
- Be sure to check the ISR and IPRP translations to ensure they are accurate.
- We have found errors in the listings of the documents.
- For example, in the listing of the cited documents in two highly related applications, the foreign language ISRs cited different documents, but both translations cited the same documents.
- If you only looked at the translations of the ISRs, you would have missed important documents cited in the original ISRs.
- Chapter II prosecution during PCT phase
- If Chapter II was entered, check the IPRP to determine if additional documents were cited by the examiner.
- Includes:
- Affirmative misrepresentations of material fact;
- Failure to disclose material information; or
- Submission of false material information;
- With an intent to deceive.
- If you consider a document, but ultimately do not cite it in an IDS:
- Place a memo in the file that indicates why you decided not to cite it (e.g., document is not material or is cumulative). MPEP 2004(18).
- Could serve as evidence of no intent to deceive.
- In Therasense, the Federal Circuit established that the materiality of a withheld reference is assessed using a “but-for” materiality test (Therasense, Inc. v. Becton, Dickinson & Co., 649 F.3d 1276 (Fed. Cir. 2011)). The court must determine whether the PTO would have allowed the claim if it had been aware of the undisclosed reference.
- The USPTO published a proposed rulemaking to amend 37 C.F.R. § 1.56 to adopt the Therasense but-for materiality test (81 Fed. Reg. 74987; October 28, 2016). The comment period closed in December, 2016.
This article appeared in the 2020 Patent Prosecution Tool Kit.
Related Services

Receive insights from the most respected practitioners of IP law, straight to your inbox.
Subscribe for Updates