A Maddening Habit
Biotechnology is an unpredictable science. When its practitioners wish to enforce their patents, they often run into a serious problem. Unlike chemists or pharmacologists, who for a century or so have used chemical formulas to describe their inventions, biotechnologists, who claim new biological molecules or their uses, have a maddening habit of giving them proper names, such as “interferon,” “CD20,” or “antiretroviral agent.” The problem is that the names they use today to describe and claim their entities may well—due to rapid scientific developments—acquire a different meaning years from today, when the patent holders are ready to assert their rights. Frequently, the proper name used at filing to denote a specific molecule has become, at infringement time, a category of molecules.
The classic example is the term “interferon,” which, on the 1958 priority date of his patent application, Dr. Isaacs, the inventor, thought to be one antiviral molecule found in chicken and mice; today, the same word, “interferon,” is used to denote a genus of over 35 different molecules classified into three different types (alpha, beta and gamma) and multiple subtypes. So, what happens to a claim that was filed in 1958, that says, “A method of treating viral infections which comprises administering to a patient in need thereof an antivirally-effective amount of interferon”? What if (assuming an extremely long patent life) such a claim is asserted today against some “interferon” that was not even known to exist on the priority date? Is the claim literally infringed? Is it invalid for lack of enablement? Something in between?
Short of being all-prescient seers and predicting that the name given a species at filing will become a genus at infringement, are there any strategies that patent applicants (and their prosecution counsel) can use, that will give them some hope of dominating later discovered (yet highly similar) embodiments at infringement time? Is there any way to draft patent applications and prosecute their claims, so as to convince a court years later to interpret the claims broadly enough to capture embodiments discovered after the priority date? Those are the questions we will try and answer in this article.
The Case Law on After Discovered Embodiments
Before we get going, it is worth a quick detour to explain the basic case law on capturing After-Discovered Embodiments (ADEs). Historically, and depending on the foreseeability of the ADE on the priority date, the decisions split into two branches, and one of these further into two more sub-branches.
• First Branch. ADE is foreseeable on the priority date: claim held invalid. In cases where the ADE is foreseeable but neither enabled nor described on the priority date and the language of the claim (as construed on the infringement date) is broad enough to literally read on the accused ADE, the claim is held invalid under 35 USC § 112(a). Examples are Plant Genetic Systems v. DeKalb (Fed. Cir. 2003); Monsanto v. Syngenta (Fed. Cir. 2007); and AbbVie Deutschland v. Janssen (Fed. Cir. 2014).
Abbvie Deutschland is illustrative of this First Branch. The main claim was, “A neutralizing isolated human antibody…that binds to human IL-12 and disassociates from human IL-12 with a koff rate constant of 1×10-2s-1 or less…” The CAFC held the claim invalid based on insufficient written description of the broad genus of antibodies with the claimed koff rate constant. There was only sufficient description of one subgenus of 300 human antibodies (VH3-type), but it was not representative of another foreseeable subgenus (VH5-type, also within the claim) that encompassed the accused, after-discovered antibody. The broad claim in AbbVie Deutschland was held invalid for being broader than the written description.
• Second Branch. ADE is unknown or unforeseeable on priority date: claim saved.
In these circumstances, where the patent applicant is unknowing, the courts are more forgiving than in the First Branch, and generally do not invalidate the claim. This branch splits into two sub-branches:
- Second Branch, Sub-branch (1): Claim construed narrowly and no literal infringement. In most cases where the ADE is not known or foreseeable on the priority date and the language of the claim (as it might be construed on the infringement date) is broad enough to read on an accused ADE, the claim is construed narrowly and limited in scope to the embodiments that are enabled and described at the priority date; in consequence, the courts find no literal infringement. Examples are Genentech v. Wellcome (Fed. Cir. 1994); Schering v. Amgen (Fed. Cir. 2000); Amgen v. Hoechst Marion Roussell (Fed. Cir. 2003) and Biogen IDEC v. Glaxo (Fed. Cir. 2013)).
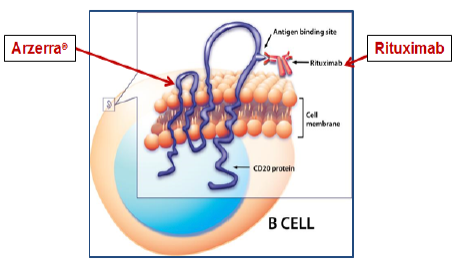
Biogen IDEC v. Glaxo is illustrative. The claim was for a method of treating chronic lymphocytic leukemia (CLL) in a patient, including the limitation “…administering to the patient an anti-CD20 antibody…” The invention was based on using, as therapy, an anti-CD20 antibody, Rituximab, which, at the filing date, was known to bind the antigen CD20 that appeared on the cell membranes of lymphoma cells. It was later discovered that Rituximab bound to just one of at least two different epitopes on CD20, the one now known as the “large loop”; no other epitope was known at the filing date or described in the specification. The claim was asserted against the ADE antibody Arzerra®. The accused Arzerra® is also an anti-CD20 antibody but it binds to a different epitope, the so-called “small loop” epitope, discovered after the filing date. The following Figure illustrates both antibodies binding to the same antigen, although to different epitopes:While Biogen IDEC, alleging literal infringement, relied on their broad claim language, “anti- CD20 antibody,” the CAFC held that “anti-CD20” meant “rituximab and antibodies that bind to the same epitope of the CD20 antigen with similar affinity and specificity as rituximab,” basing its conclusion on prosecution history disclaimer. The narrowly construed claim survived but was not literally infringed by Arzerra®.
- Second Branch, Sub-Branch (2): Claim construed broadly and literal infringement. Rare are the decisions where the ADE is not known or foreseeable on the priority date, the language of the claim (as construed on the infringement date) is broad enough to read on the ADE, and yet the court finds literal infringement. Examples are U.S. Steel v. Phillips (Fed. Cir. 1989); Scripps v. Genentech (Fed. Cir. 1991); and Stanford v. Roche (D. Ct. ND CA. 2007)). These cases may hold lessons for us.
Stanford v. Roche, although a decision of a District Court, is illustrative. The claim here was for a method of evaluating the effectiveness of anti-HIV therapy using a broad “antiretroviral agent.” The court found the claim term “antiretroviral agent” to be generic, even though the only such agents known on the 1992 filing date were reverse transcription inhibitors. It held that the claim captured the accused ADE protease inhibitors, whose use was invented in 1995–1996. Roche’s argument that “antiretroviral agent” should be defined as limited to agents available for the treatment of AIDS/HIV infected patients in 1992, was rejected. The claim term was held to read on a broader genus of agents, not just on the subgenus of reverse transcription inhibitors.
A Multi-Million Dollar Question
The multi-million dollar question to inventors and their lawyers is: How do you avoid invalidity of the claim (as in the First Branch), save it (as in the Second Branch), and get it construed broadly so as to capture the ADE as literally infringed (as in the Second Branch, Sub-Branch (2))? Put differently, how do you avoid AbbVie Deutschland and Biogen IDEC, yet catch the golden ring of Stanford v. Roche? While this is not easy, it may be doable with careful drafting and prosecution. Here are some lessons gleaned from the case law.
Lesson Number 1. Even after the first filing date, an objectively unknowing inventor should stay up on developments in the technology and re-file as necessary.
Obviously, if an inventor or her attorney do not know of or cannot foresee ADEs, they will be spared the sting of patent invalidity for lack of enablement. The inventors in Biogen IDEC did not lose their claims, even though they failed to describe and enable antibodies to the small loop of CD20. Their claims survived, although in narrow, not literally infringed, fashion. However, even narrow survival means the possibility of asserting the Doctrine of Equivalents in litigation. Thus, while objective ignorance leads to claim survival, it is with a major qualification: One cannot be willfully ignorant, putting one’s head in the sand like an ostrich. When the court asks what was known or foreseeable on the priority date, it will always use an objective standard: that of the POSITA, the person of ordinary skill in the art. An inventor will be charged with knowing everything there is to know on the filing date, so she might as well actually know it. Then, as the technology develops further and ADEs appear in the literature, she can update every subsequent re-filing, as in a new provisional or CIP. If, say, a new epitope has been discovered, it may be worthwhile filing a CIP to describe it as another example within the broad genus claim; this may allow a judge at infringement time to broadly interpret a term like “CD20 antigen” so as to encompass both epitopes. (A claim to the specific new ADE will have a new priority date; we are discussing here preserving the original date for the generic claim.)
Lesson Number 2. If, at the priority date, an inventor foresees other embodiments, especially of a different type than the ones exemplified, he should mention them as part of the first filed generic description, try and reduce at least one example to practice, and file a subsequent application.
If, at the priority date, the inventor can foresee the existence of not yet discovered embodiments, such as the different antibody chains of the VH5-type in AbbVie Deutschland, he should at least mention this in the patent application as a prophetic description of a broader genus. Ideally, the inventor should later try and reduce such embodiment to practice and add it in a new provisional or CIP application. This addition would be a means to confirm his earlier prediction of a broad genus, and support a generic claim without losing the earlier priority date.
Lesson Number 3. An inventor should always try and use in her original specification language of category, not of species or narrower subgenus.
Even if an all-knowing and prescient inventor stays up on the literature but is not able to foresee any future embodiments, she should always assume that she has invented a group of molecules, not just one. As in Stanford v. Roche, she should use language of broad category at the filing date: not “reverse transcription inhibitors” but “antiretroviral-agents.”
Lesson Number 4. The attorney should include claims of different scope and not drop them during prosecution.
The applicant in AbbVie Deutschland failed to include and maintain a dependent claim drawn to the distinct class of 300 VH3-type human antibodies that he had made. All he did was claim the broadest genus functionally, by koff rate. Had he included a dependent claim drawn to the VH3-type he fully described, such a claim would have survived invalidity for lack of written description. Then, during litigation, it may have been held to be infringed by the accused VH5 antibodies under the Doctrine of Equivalents. There was no such claim in the AbbVie patent and it never happened.
Lesson Number 5. Both inventor and attorney should try and glean a “principle of the invention” and include it in the specification.
There is a great case, U.S. Steel Corp. v. Phillips Petroleum Co. (Fed. Cir. 1989), that should help unknowing biotech inventors who have made pioneering inventions. The claim was, “Normally solid polypropylene, consisting essentially of recurring propylene units, having a substantial crystalline polypropylene content.” The CAFC looked beyond the literal words of the claim, focused on the so-called “principle of the invention” (which it decided was “high crystallinity”), and held that an ADE of even higher crystallinity (unforeseeable at the priority date), infringed literally. Both the claimed and accused polymers shared the same “high crystallinity” principle said the court, and it remained unchanged in the accused product.
Given the advantage of relying on a “principle,” and even if it may be difficult to glean one from the discovery of a single biological species, we recommend describing such a principle in the specification. If a smart scientist or attorney can understand a principle (e.g. “crystallinity” or “CLL sensitivity to anti-CD20 antibodies”), they should describe it. Invoking a principle may lead to broad claim construction and allow the capture of ADEs by literal infringement. Even if the claim is construed narrowly, the description of a principle may assist in achieving success under the Doctrine of Equivalents (which, in its modern three- part test, is but a refinement of its 19th Century form, i.e., “comparing the principles of the claimed and accused devices”).
Lesson Number 6. Use Means-plus-function and Think Outside The Box
Let us propose one more rather unconventional approach to capturing ADEs by careful claim drafting: the use of means plus function claims under 35 U.S.C. § 112 (f) (previously known as 6th paragraph). In 2014, in Williamson v. Citrix Online, LLC, the CAFC held that the absence of the words “means for” does not preclude applying means plus function analysis; there is no longer a strong presumption to that effect. This development may help biotechnology holders – who rarely, if ever, use claims in means plus function format – achieve a generic interpretation of its claims. This is especially the case when the situation is one where the “means” described in the specification at the filing date has changed by the time of infringement. And, if the prosecuting attorney can include a means plus function claim, the better.
Assume that the Biogen IDEC inventors had obtained a claim as follows, “A method for treating CLL in a human patient, comprising the step of administering to the patient an effective amount of antibody means to bind to CD20, so as to treat the CLL…” The term “administering antibody means…” could (and should) be construed to cover the “corresponding…material…described in the specification and equivalents thereof.” The material described in the specification is Rituximab, the existing antibody against the large loop epitope of CD20. The focus then is on the statutory phrase “and equivalents thereof.” The claim construction argument to be made in court is that the claim term “antibody means” should be construed to cover all manners of antibodies that bind to CD20 that act in an equivalent manner to Rituximab. Under established case law from the CAFC, the structural equivalency test under 35 U.S.C. § 112 (f) is a “way-result” two-part test; the function of the accused structural equivalent needs to be identical to that of the “corresponding…material…described in the specification…” Thus, the alleged structural equivalent of the described Rituximab (i.e., the accused antibody Arzerra®), while performing the identical function, must do so in substantially the same manner, and must achieve substantially the same result as Rituximab. If Biogen IDEC could convince the court that the claim should be construed broadly enough to read on antibodies that bind to different epitopes of CD20, including some to the small loop, and provided that the two-way test is met, the patentee would have a claim that is literally infringed. That is the golden ring of capturing ADEs.
A more in-depth analysis of this topic can be found in Goldstein, J.A., 25 Fed. Cir. Bar J. No. 3, 401-451 (2016).
This article appeared in the 2020 Patent Prosecution Tool Kit.
Related Industries
Related Services

Receive insights from the most respected practitioners of IP law, straight to your inbox.
Subscribe for Updates